Because its OH bond is weaker, \(FSO_3H\) is a stronger acid than sulfuric acid. The predicted order of acid strengths given here is confirmed by the measured pKa values for these acids: \[pKa H_2SO_3 1.85<H_2SO_4^−2 < FSO_3H−10 \nonumber\] The structures of both trifluoramine and hydroxylamine are similar to that of ammonia.
Solved Rank the indicated protons in decreasing order of | Chegg.com
Jul 1, 2023Generally, the following trends can help you rank protons in order of increasing acidity: Electronegativity: Protons bonded to more electronegative atoms tend to be more acidic. This is because electronegative atoms can withdraw electron density from the proton, making it easier to remove.

Source Image: brainly.com
Download Image
2,184 solutions chemistry Which of the following species has the largest dipole moment (i.e., is the most polar)? A.CH3Cl B.CH4 C.CH3F D.CH3Br chemistry Select the most basic compound from the following: a) \ce Sb2O5 SbX 2 OX 5 b)

Source Image: chegg.com
Download Image
Solved Rank the indicated proton with its acidity, using 1 | Chegg.com Rank the labelled protons in order of increasing acidity. d < c < b < e < a. Rank the labelled protons in order of increasing acidity. b < a < c. Rank the following compounds in order of decreasing acidity. C < A < B. Rank the following compounds in order of increasing basicity. Resonance

Source Image: chegg.com
Download Image
Rank The Indicated Protons In Order Of Increasing Acidity
Rank the labelled protons in order of increasing acidity. d < c < b < e < a. Rank the labelled protons in order of increasing acidity. b < a < c. Rank the following compounds in order of decreasing acidity. C < A < B. Rank the following compounds in order of increasing basicity. Resonance SOLVED:Rank the labeled protons in each compound in order of increasing acidity. Organic Chemistry Janice Gorzynski Smith 4 Edition Chapter 23, Problem 35 Question Answered step-by-step Rank the labeled protons in each compound in order of increasing acidity. Video Answer Solved by verified expert Ian K. Numerade Educator Like View Text Answer
Solved On the structure shown below, rank the indicated | Chegg.com
Organic Chemistry I (Cortes) 11: Bronsted Acid-Base Chemistry Lesson 6.8: pH and Color Change – American Chemical Society

Source Image: acs.org
Download Image
Solved Question 26 Rank the indicated protons in the order | Chegg.com Organic Chemistry I (Cortes) 11: Bronsted Acid-Base Chemistry
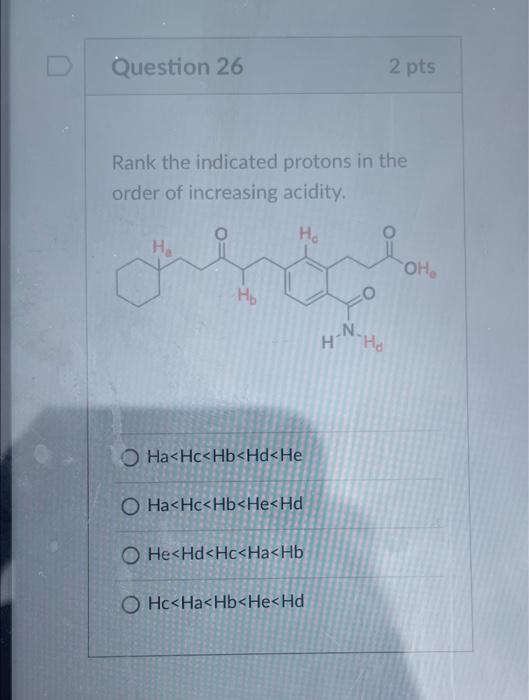
Source Image: chegg.com
Download Image
Solved Rank the indicated protons in decreasing order of | Chegg.com Because its OH bond is weaker, \(FSO_3H\) is a stronger acid than sulfuric acid. The predicted order of acid strengths given here is confirmed by the measured pKa values for these acids: \[pKa H_2SO_3 1.85<H_2SO_4^−2 < FSO_3H−10 \nonumber\] The structures of both trifluoramine and hydroxylamine are similar to that of ammonia.

Source Image: chegg.com
Download Image
Solved Rank the indicated proton with its acidity, using 1 | Chegg.com 2,184 solutions chemistry Which of the following species has the largest dipole moment (i.e., is the most polar)? A.CH3Cl B.CH4 C.CH3F D.CH3Br chemistry Select the most basic compound from the following: a) \ce Sb2O5 SbX 2 OX 5 b)
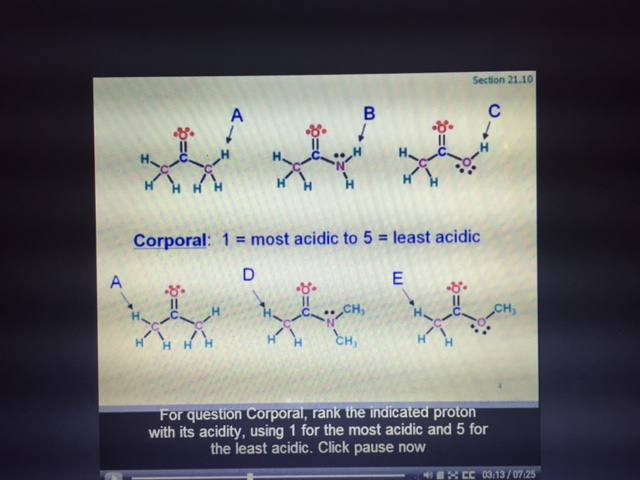
Source Image: chegg.com
Download Image
Solved Question 25 4 pts Which sequence ranks the indicated | Chegg.com Ask Question Asked 7 years, 4 months ago Modified 3 years, 9 months ago Viewed 5k times 0 I have an acid and base organic chem quiz tomorrow and I need help determining how acidic protons are. I understand the concept of atoms, resonance, induction, and orbital when considering the acidity of protons.
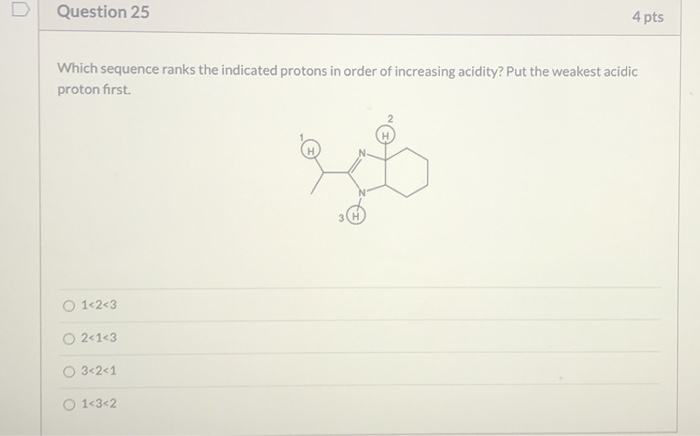
Source Image: chegg.com
Download Image
Rank the protons in the labeled CH2 groups in order of increasing acidity, and explain why you chose this order. | Homework.Study.com Rank the labelled protons in order of increasing acidity. d < c < b < e < a. Rank the labelled protons in order of increasing acidity. b < a < c. Rank the following compounds in order of decreasing acidity. C < A < B. Rank the following compounds in order of increasing basicity. Resonance

Source Image: homework.study.com
Download Image
7.02 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as 146C to describe particular nuclei – TutorMyself Chemistry SOLVED:Rank the labeled protons in each compound in order of increasing acidity. Organic Chemistry Janice Gorzynski Smith 4 Edition Chapter 23, Problem 35 Question Answered step-by-step Rank the labeled protons in each compound in order of increasing acidity. Video Answer Solved by verified expert Ian K. Numerade Educator Like View Text Answer
Source Image: tutormyself.com
Download Image
Solved Question 26 Rank the indicated protons in the order | Chegg.com
7.02 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as 146C to describe particular nuclei – TutorMyself Chemistry Jul 1, 2023Generally, the following trends can help you rank protons in order of increasing acidity: Electronegativity: Protons bonded to more electronegative atoms tend to be more acidic. This is because electronegative atoms can withdraw electron density from the proton, making it easier to remove.
Solved Rank the indicated proton with its acidity, using 1 | Chegg.com Rank the protons in the labeled CH2 groups in order of increasing acidity, and explain why you chose this order. | Homework.Study.com Ask Question Asked 7 years, 4 months ago Modified 3 years, 9 months ago Viewed 5k times 0 I have an acid and base organic chem quiz tomorrow and I need help determining how acidic protons are. I understand the concept of atoms, resonance, induction, and orbital when considering the acidity of protons.